WASHINGTON – The Association for Accessible Medicines has warned that the Inflation Reduction Act (IRA) inadvertently harms competition from lower-cost generic and biosimilar medications. Despite claims that the IRA’s drug price negotiation process would not impact these markets, recent developments reveal that the law’s structure actively undermines competition, potentially delaying patient access to affordable medications.
One of the last acts of the Biden Administration was selecting 15 drugs for price negotiation under the IRA. However, AAM points out that 13 of these drugs already have generic or biosimilar competitors either approved or in the pipeline. This timing exposes a critical flaw in the IRA: price controls are applied before generics or biosimilars can enter the market.
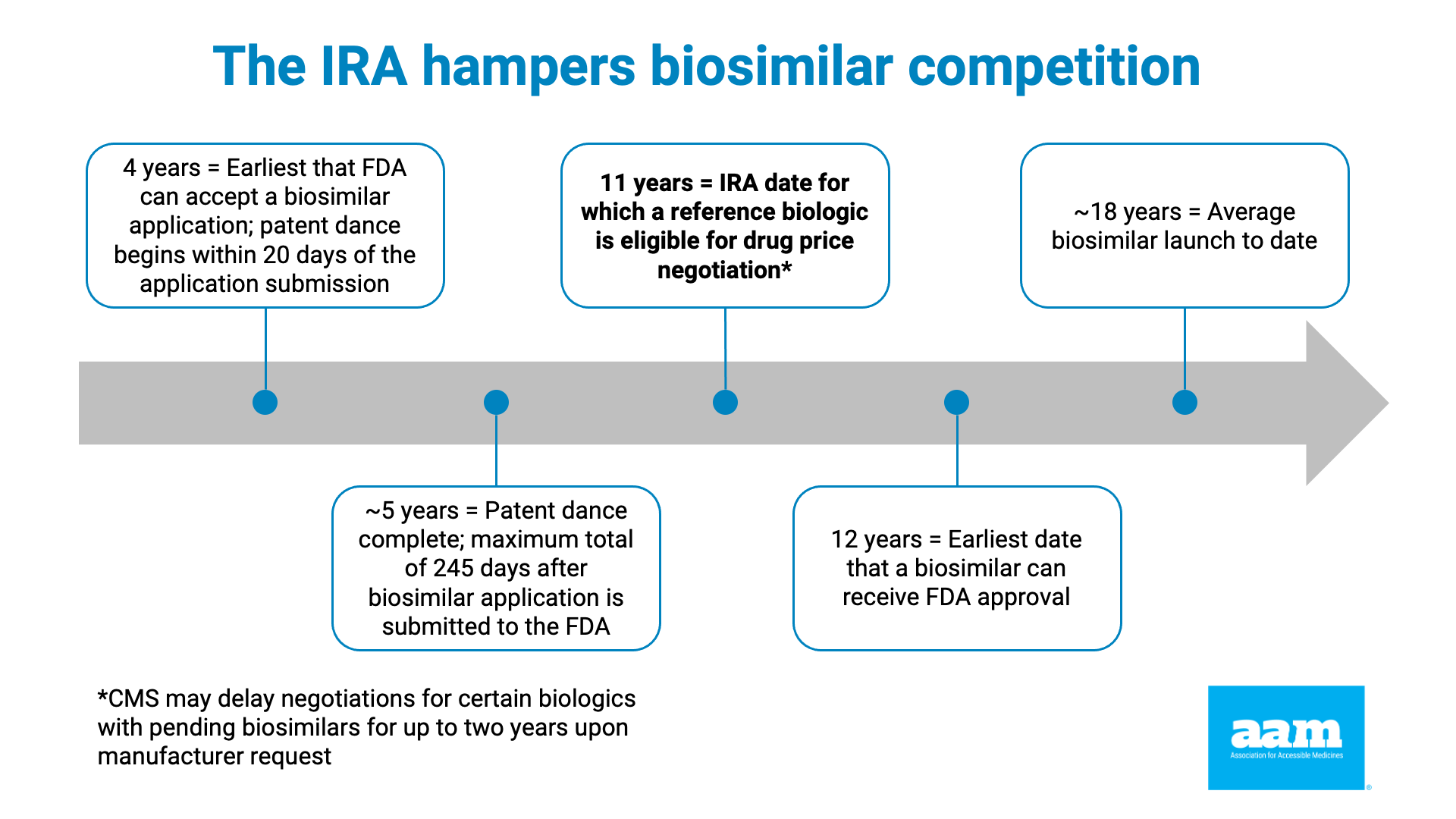
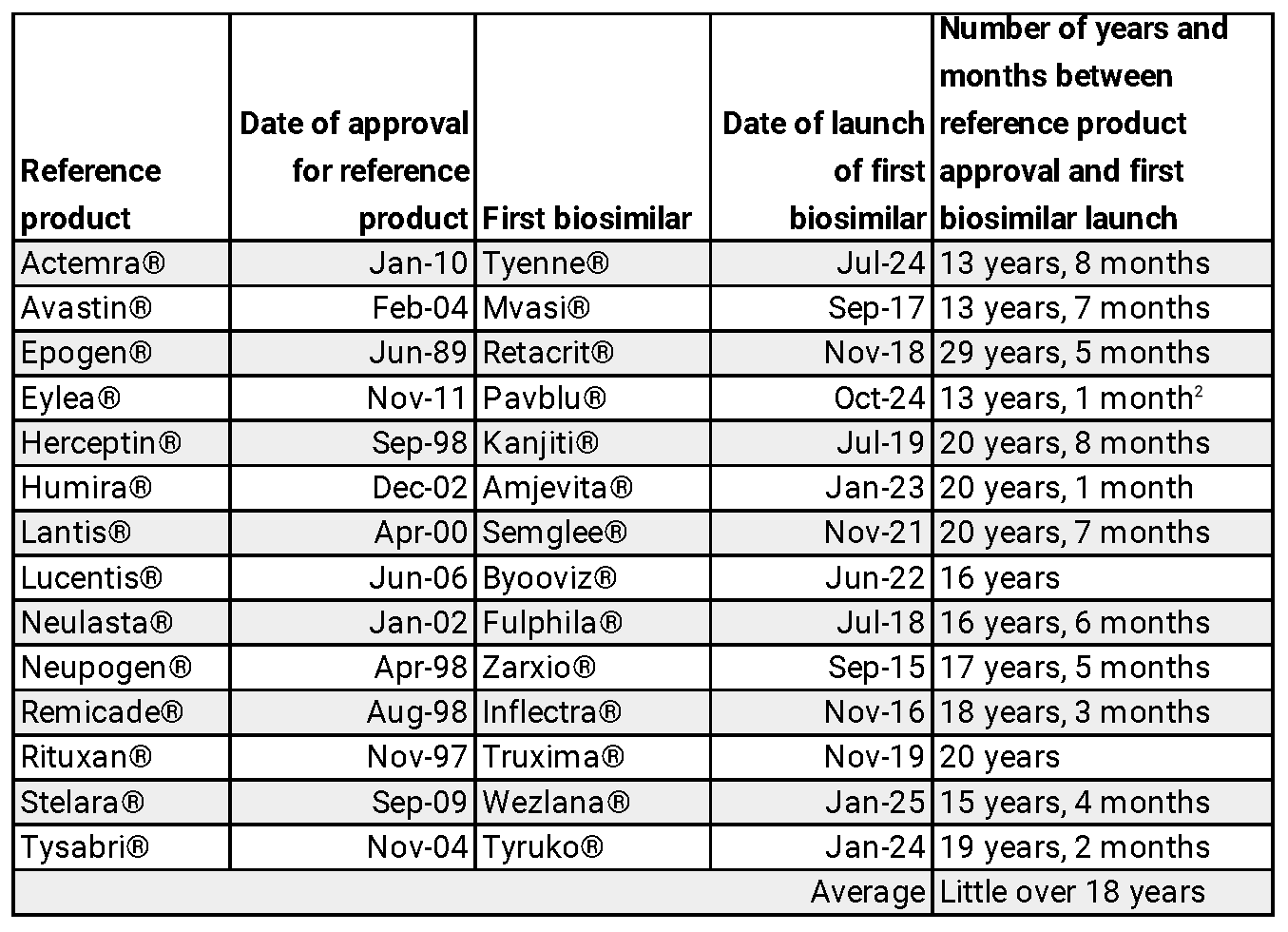
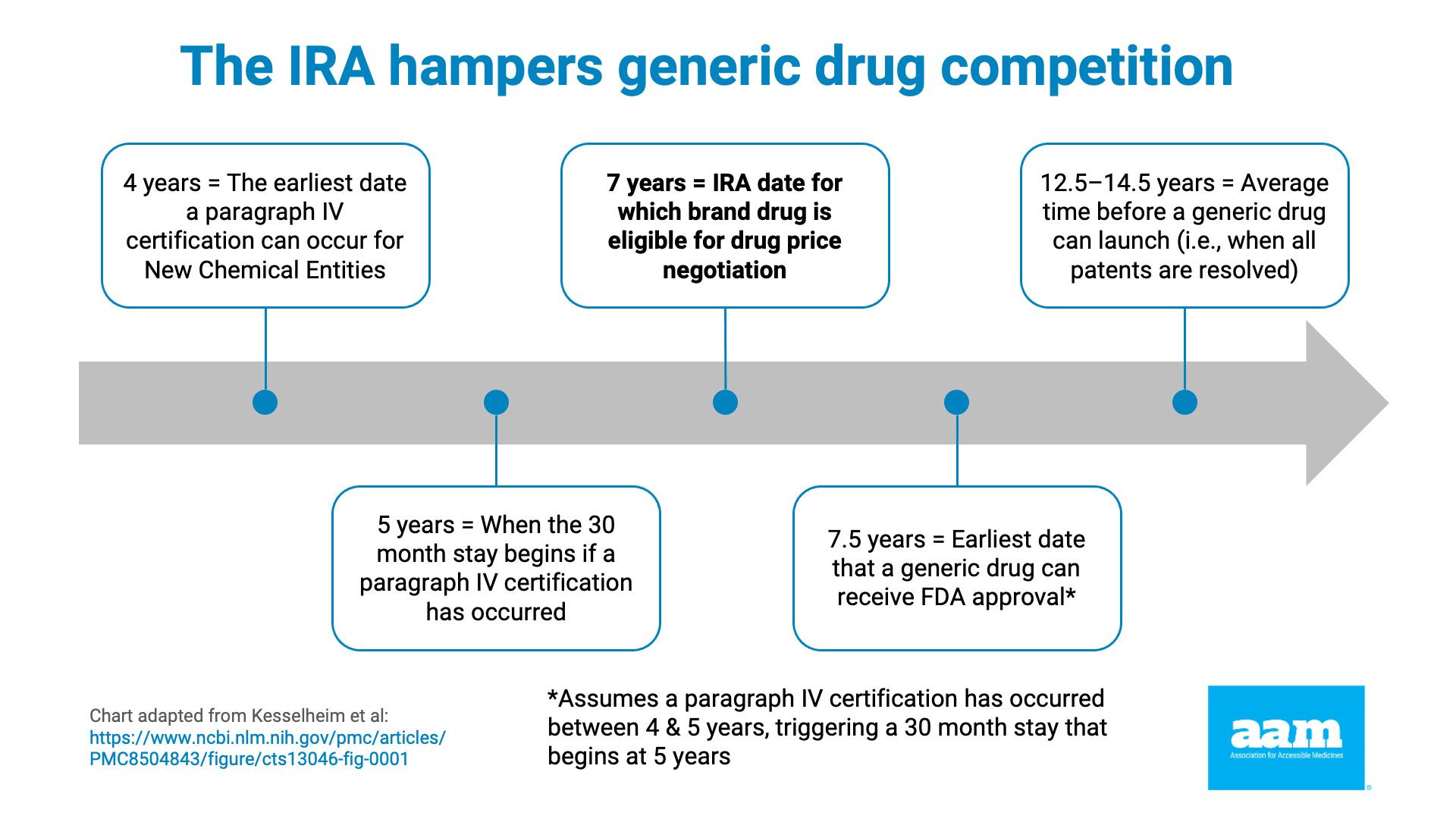
Under the IRA, price negotiations begin when a brand-name drug has been on the market for 7 years (or 11 years for biologics). Yet data shows that the average market entry time for a first generic is 12-14 years and over 18 years for biosimilars. This misalignment means price controls are imposed just as lower-cost alternatives are poised to launch, effectively stifling competition.
The AAM also criticized the IRA’s impact on existing competition. Under the law, Medicare drug plans must cover brand-name drugs subject to price controls, even if lower-cost generics or biosimilars are available. While the Centers for Medicare & Medicaid Services (CMS) allows plans to substitute a brand drug with a newly available generic or biosimilar, this provision creates skewed incentives.
For example, Stelara (ustekinumab), a drug selected for IRA negotiation, already faces competition from biosimilars like Wezlana, with five more expected in 2025. However, Medicare plans must still cover Stelara at the negotiated price, even if biosimilars are cheaper. This disincentivizes manufacturers from launching lower-cost alternatives promptly, as delayed launches could maximize market share under the IRA’s rules.
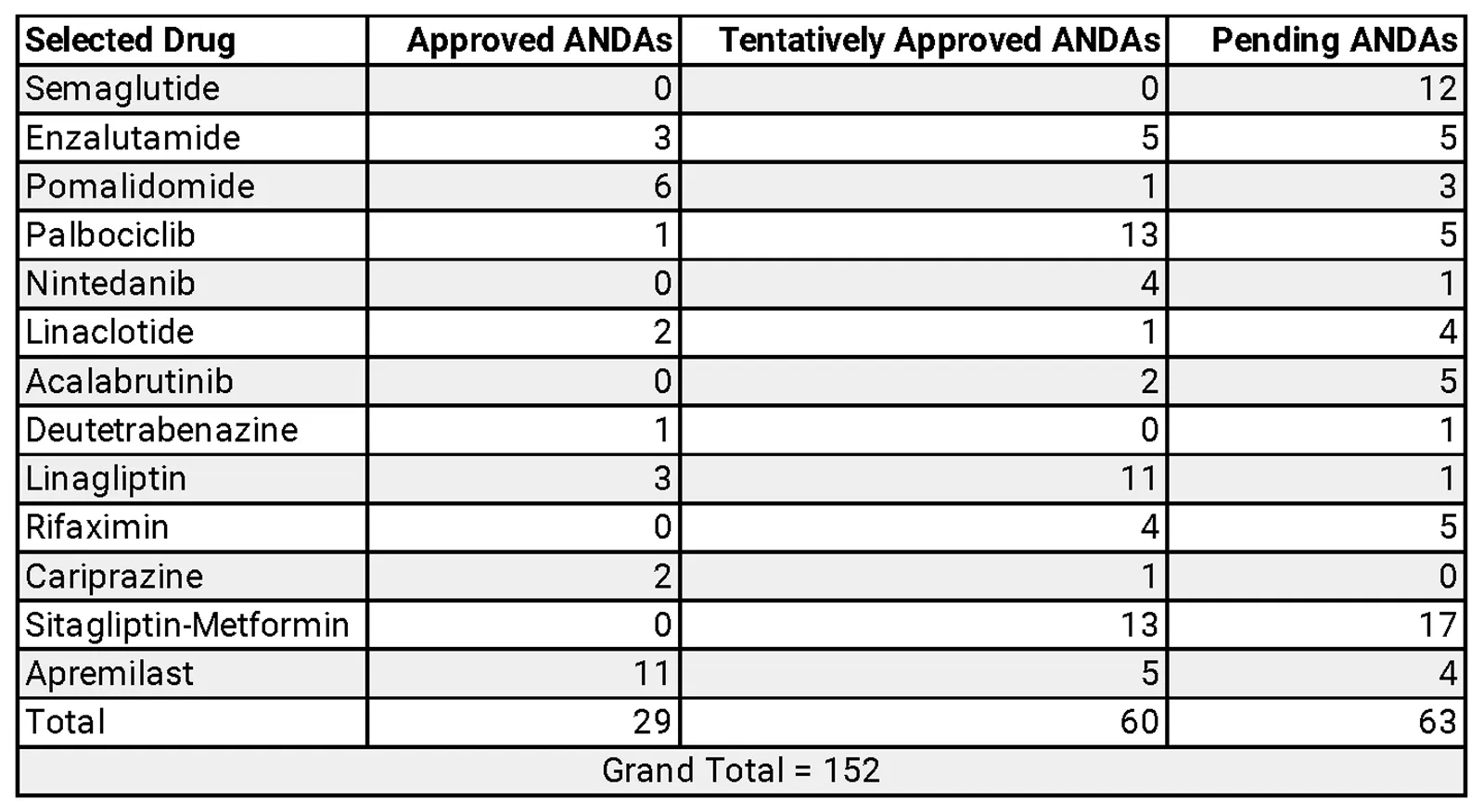
The AAM warns that the IRA’s price controls could lead to fewer generics and biosimilars entering the market, slowing competition and keeping drug prices high for longer. A recent IQVIA Institute analysis suggests this slowdown is already underway.
By creating uncertainty and discouraging investment in generics and biosimilars, the IRA may inadvertently extend brand monopolies, harming patients, taxpayers, and employers. While Medicare beneficiaries may see some price reductions, reduced competition could raise costs in the broader market.
The AAM urges policymakers to reform the IRA to address these unintended consequences. Without changes, the law risks undermining the competition it was designed to promote, leaving patients without access to the cost savings of generics and biosimilars.