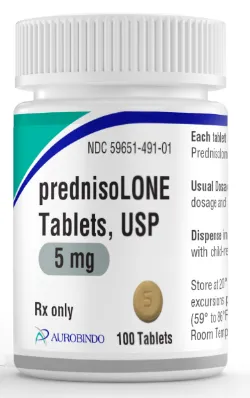
EAST WINDSOR, N.J. – Aurobindo Pharma has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application for Prednisolone Tablets USP, 5mg.
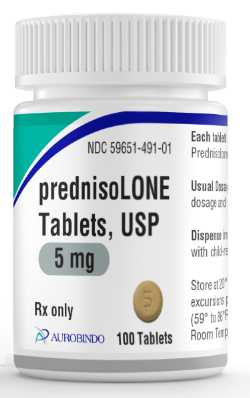
Aurobindo Pharma’s prednisolone tablets, are an AB-rated generic equivalent to the reference listed drug (RLD), Prednisolone (Millipred) Tablets USP, manufactured by Watson Laboratories Inc.
Prednisolone tablets, are indicated in the following conditions.
1. Endocrine disorders
2. Rheumatic disorders
3. Collagen diseases
4. Dermatologic diseases
5. Allergic states
6. Ophthalmic diseases
7. Respiratory diseases
8. Hematologic disorders
9. Neoplastic diseases
10. Edematous states
11. Gastrointestinal diseases
12. Nervous system
13. Tuberculous meningitis with subarachnoid block or impending block when used concurrently with appropriate antituberculous chemotherapy; trichinosis with neurologic or myocardial involvement.
Prednisolone ablets, has an estimated market size of US $3.2 Million for the twelve months ending March 2023, as per IQVIA.