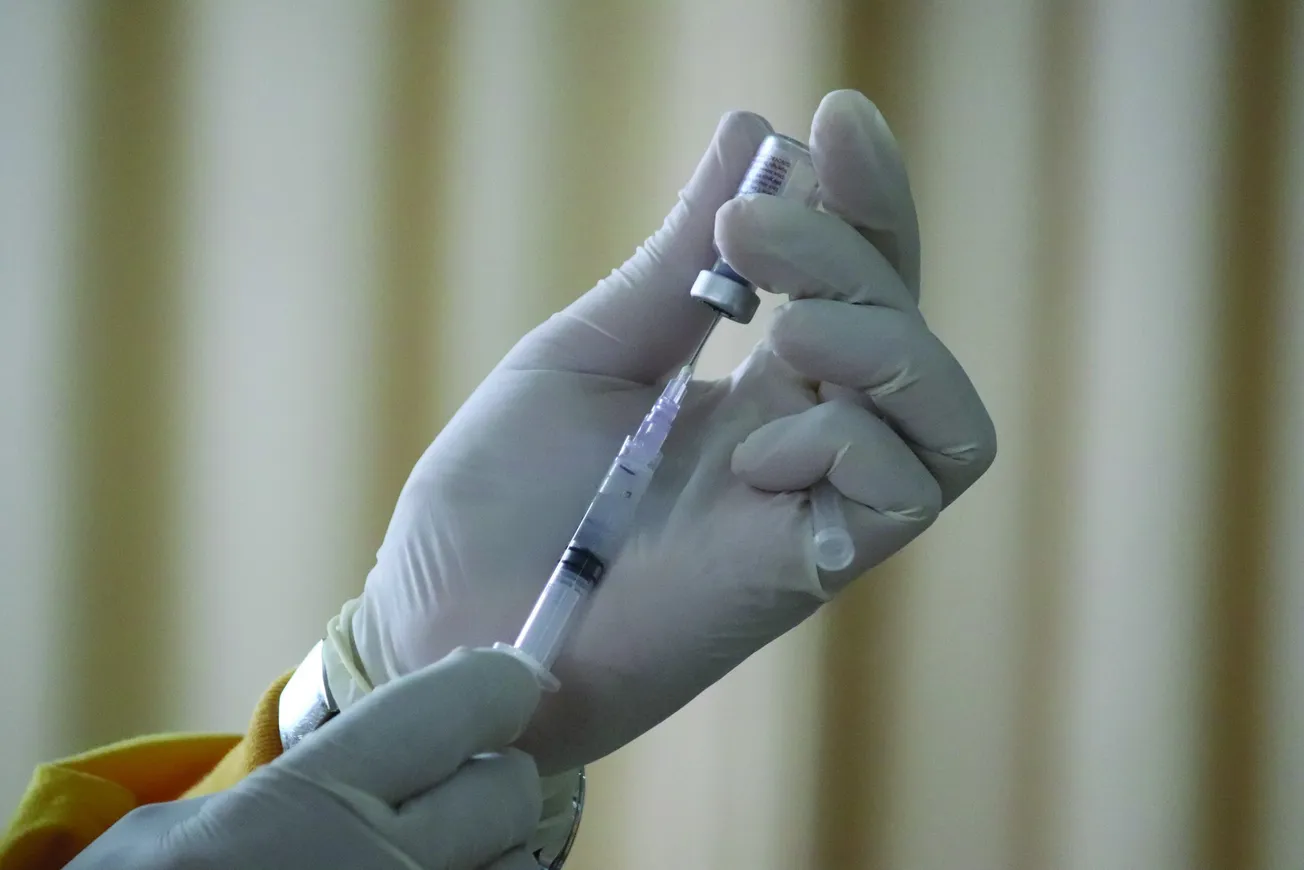
WASHINGTON — The Food and Drug Administration approved a new round of vaccines against COVID-19.
The vaccines from Moderna and Pfizer and its partner BioNTech were approved Monday for people 12 and older and under an emergency use authorization for children ages 6 months to 11 years old.
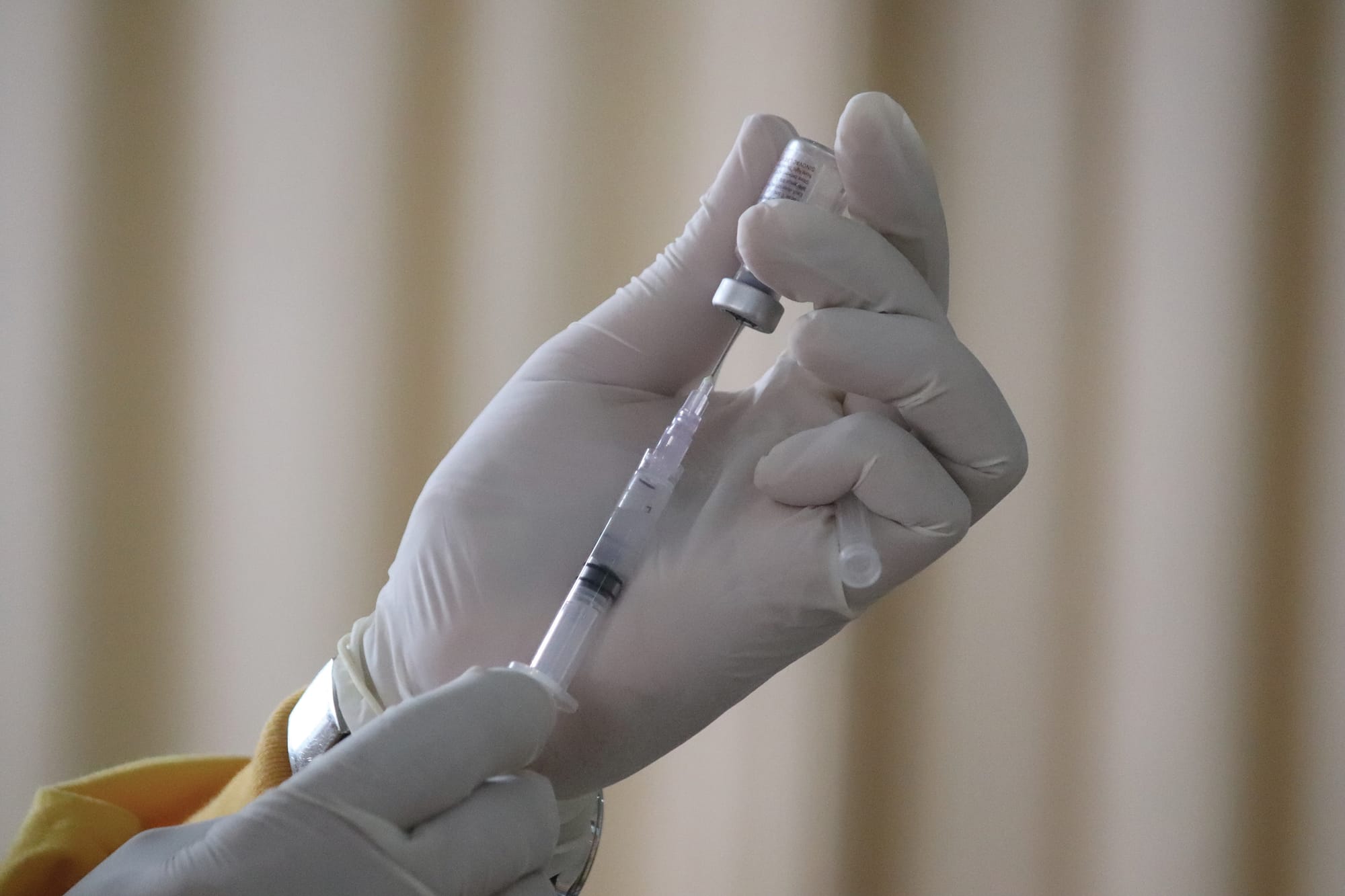
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” said Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
The vaccines target the omicron subvariant called XBB.1.5, which is no longer the most common strain in circulation. The vaccine makers and FDA say that the vaccine should still provide good protection against COVID. Recent studies support that.
The shots would be given as a single dose for most people 5 years of age and older, regardless of prior COVID-19 vaccination history.
Children younger than 5 may be eligible for multiple doses of this season’s vaccine if they had not previously finished a three-dose series with earlier COVID-19 vaccines.
Moderna and Pfizer say they have produced ample supplies of their vaccines.