SAN LEANDRO, Calif. — iHEAR Medical announced plans to launch advanced over-the-counter (OTC) hearing solutions in major drugstore chains and independent pharmacies across the United States this June.
The company also announced the appointment of John Luna as its chief executive officer to lead iHEAR’s expansion into mass retail, offering affordable, high quality hearing products to millions of Americans currently denied access to effective hearing solutions.
The OTC Hearing Aid Act of 2017 was recently passed into law to improve the affordability and accessibility of hearing aids. About 86% of Americans with hearing loss, over 30 million people, currently go untreated, making hearing impairment one of the most common forms of disability in the United States. The consequences of untreated hearing loss include lower income and higher incidence of depression, social isolation and cognitive decline. Recent reports have highlighted how hearing aid use can be a life changing experience for many.
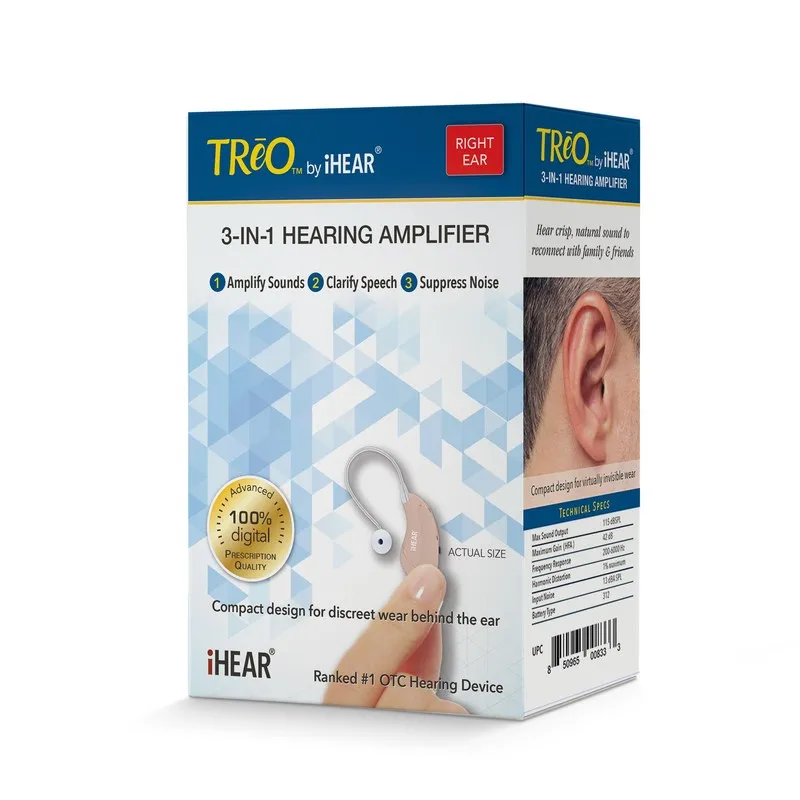
iHEAR will launch the TReO, the first prescription-quality hearing amplifier for OTC markets, and the iHearTest kit, the first FDA-cleared home hearing screener, in 500 drugstores in June 2018. The launch will expand to over 1,300 stores by the end of 2018, including major drugstore chains and independent pharmacies served by leading wholesalers.
The TReO will retail for $299, compared to $2,400 on average for comparable programmable hearing devices sold in traditional hearing aid centers. The iHearTest will retail for $69 and is eligible for Flexible Spending Account reimbursement.