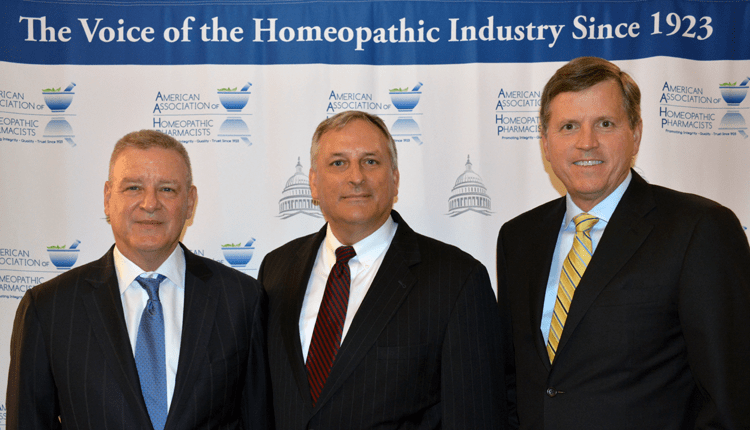
Emerson Group CEO Scott Emerson, AAHP president Mark Land and CHPA president and CEO Scott Melville

Attendees heard about trends affecting manufacturers and retailers of homeopathic medicines
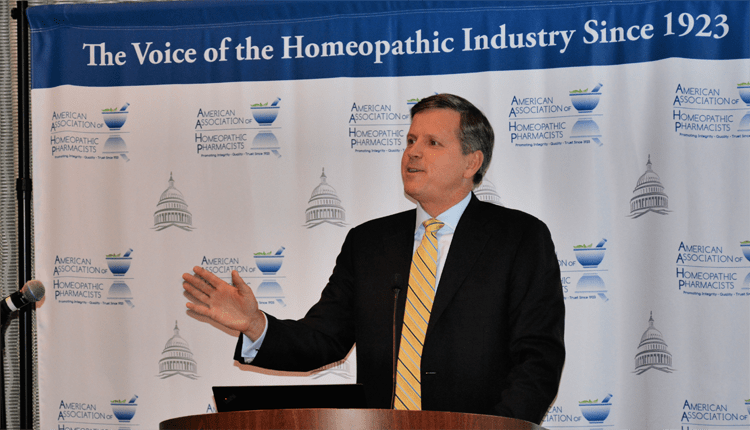
Scott Melville of the Consumer Healthcare Products Association

The American Association of Homeopathic Pharmacists (AAHP) convened its inaugural industry summit in Baltimore recently

Wegmans’ Karen Shadders with AAHP president Mark Land
BALTIMORE — The American Association of Homeopathic Pharmacists (AAHP) convened its inaugural industry summit here last month to celebrate recent gains in the marketplace while assessing the challenges suppliers face in engaging with regulators and earning consumers’ trust.
“We have a responsibility to produce products of the highest quality so that consumers and families across the United States and the world receive the full health benefits of homeopathy without safety risks or questionable reliability,” said Mark Land, president of AAHP.
Sales of homeopathic drugs through brick-and-mortar stores doubled over the past six years, to $517 million in 2018, said Scott Emerson, founder and chief executive officer of the Emerson Group, which works with over-the-counter health and beauty brands to grow sales. In 2015, mass retailers surpassed natural food stores and the internet as the largest outlet for homeopathic products, accounting for about half of total sales, Emerson said. “Customers are buying natural, safe and effective; they’re not buying homeopathy,” he said, citing a customer survey suggesting that 15% of women reported making a homeopathic purchase, when in fact more than a third of respondents had bought a homeopathic brand to address a specific health need.
At Wegmans Food Markets Inc., homeopathic promotion on end-caps and consumer education produced notable sales growth, said Karen Shadders, vice president for health, wellness, home and entertaining at the Rochester, N.Y.-based supermarket chain. Shadders attended the summit to accept the AAHP’s Integrative Medicine Award on behalf of Wegmans.
On the regulatory front, homeopathic suppliers are under pressure from the Food and Drug Administration to make sure products are appropriately manufactured and labeled.
In light of the homeopathic category’s growth, the FDA has proposed a risk-based enforcement approach to drug products labeled as homeopathic and marketed for serious diseases or conditions where the products haven’t been shown to offer clinical benefits, said Francis Godwin, director of the FDA’s office of manufacturing quality. “To meet the guidelines, a product must be pure, safe to use and its strength accurately represented on the label,” Godwin said.
About 85% of homeopathic manufacturing sites the FDA audits worldwide are in compliance with its guidelines, Godwin said. The agency sent 71 warning letters last year to manufacturers in the United States, China, Brazil, India and elsewhere detailing flaws in manufacturing operations and quality assurance systems, he said. Homeopathic drugs account for less than 4% of the U.S. inventory of O-T-C drugs and about 6.5% of the enforcement actions taken by the agency.









