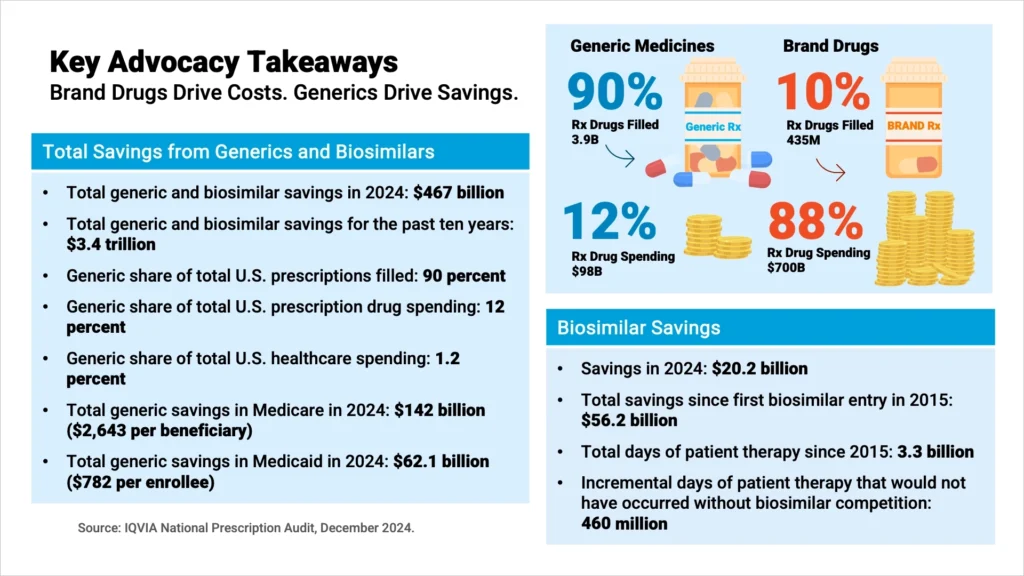
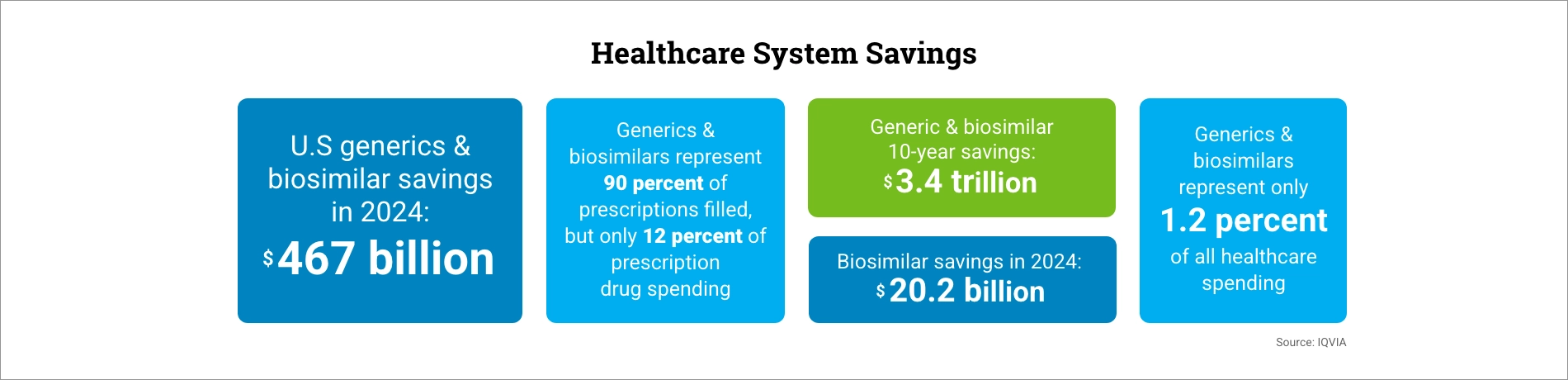
WASHINGTON — Generic and biosimilar medicines saved U.S. patients and the healthcare system $467 billion in 2024, according to the 2025 U.S. Generic & Biosimilar Medicines Savings Report released today by the Association for Accessible Medicines (AAM) and the Biosimilars Council. Over the past decade, these lower-cost alternatives have generated more than $3.4 trillion in savings.
The report highlights the outsized role generics and biosimilars play in reducing costs. They account for 90% of prescriptions filled in the U.S. but only 12% of total drug spending. By contrast, brand-name drugs represented just 10% of prescriptions but consumed 88% of spending — $700 billion compared with $98 billion for generics.
“Simply put: Generic medicines save money,” said John Murphy III, President and CEO of AAM. “Generics and biosimilars are the only sector that consistently results in decreased spending across the U.S. healthcare ecosystem. Unfortunately, right now little is being done to infuse sustainability into the generic and biosimilar marketplace. The significant price deflation of the last 30 years can lead to unsustainable market conditions for generic drug manufacturers, dangerously impacting patient care, and increasing the likelihood of shortages or even drugs leaving the market. Policymakers must streamline FDA processes, curb patent abuse, and stop PBMs and Medicare policies from denying patient access and rollback harmful federal policies – including IRA price controls.”
Medicare and Patient Impact
Savings were especially significant for Medicare beneficiaries, totaling $142 billion in 2024 — the equivalent of $2,643 per enrollee. Yet, AAM cautioned that patients are still paying too much for many generic prescriptions due to reimbursement and coverage barriers.
“The biosimilars industry celebrated a decade of pathbreaking progress, including new therapy areas, $56.2 billion in savings for patients and the healthcare system, and 3.3 billion days of patient therapy,” said Giuseppe Randazzo, Interim Executive Director, Biosimilars Council. “However, over the next decade, 118 biologics are expected to lose patent exclusivity, presenting a $234 billion opportunity for biosimilars. But of these 118 biologics, right now only 12 molecules have biosimilars in development – a ‘biosimilar void.’ Sustainability of our industry is not guaranteed – we must double down efforts to ensure the biosimilars market reaches its full potential.”
Biosimilars: A Decade of Progress, and a “Biosimilar Void”
The biosimilars sector marked 10 years since the first U.S. approval, delivering $56.2 billion in savings and supporting 3.3 billion days of patient therapy. Biosimilars have also expanded access, contributing nearly 460 million incremental days of treatment that would otherwise not have been provided.
“Over the next decade, 118 biologics are set to lose patent exclusivity, representing a $234 billion opportunity,” said Giuseppe Randazzo, Interim Executive Director of the Biosimilars Council. “But only 12 molecules currently have biosimilars in development. Without stronger policies, the U.S. risks a ‘biosimilar void’ that will limit access and erode potential savings.”
Policy Recommendations
The report calls for policymakers to:
- Streamline FDA approval processes,
- Eliminate patent abuse,
- Reform Medicare and pharmacy benefit manager (PBM) policies, and
- Reconsider provisions of the Inflation Reduction Act (IRA) that could deter competition.
Without such action, industry leaders warn that shortages, market exits, and reduced patient access could undermine the very system that delivers billions in savings each year.








