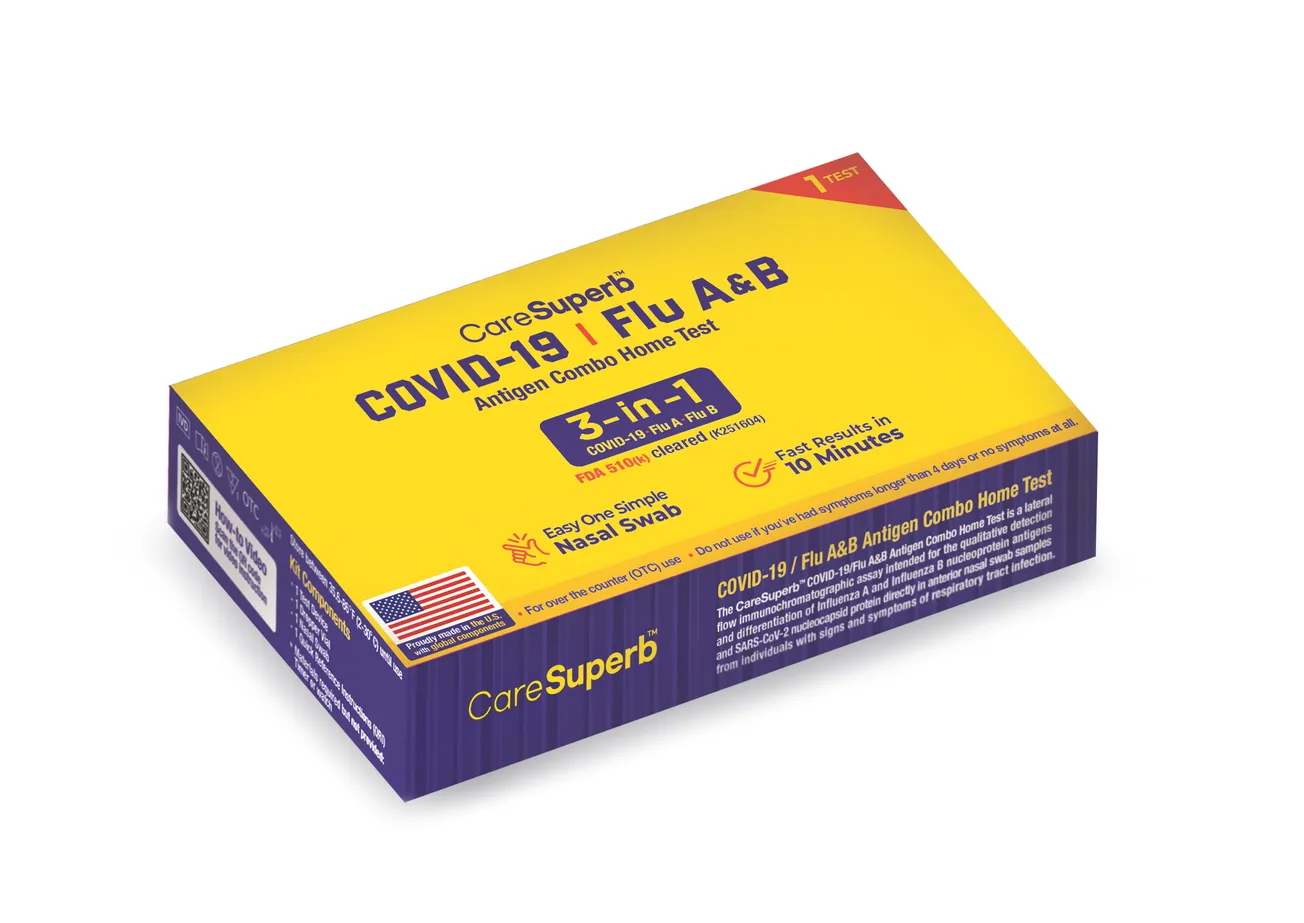
MONROE TOWNSHIP, N.J. — Access Bio, a global leader in diagnostic solutions, has expanded retail distribution across North America for its CareSuperb COVID-19/Flu A&B Antigen Combo Home Test, following FDA 510(k) clearance on August 22, 2025. The test allows consumers to detect both COVID-19 and influenza A and B from a single at-home kit—just in time for the upcoming respiratory virus season.
Since flu and COVID-19 cases usually spike between December and February, introducing CareSuperb into major retail channels ensures consumers can access reliable, affordable testing made in the U.S. The test’s simple and clear design makes it especially suitable for consumers who want an easy-to-use, intuitive experience that reduces confusion and helps guide care decisions quickly.
Instructions for Use CareSuperb™ COVID-19/Flu A&B Antigen Combo Home Test
Designed for Everyday Consumers
The CareSuperb Combo Kit achieved a 96.5% usability score during FDA clearance studies—the highest among manufacturers submitting for review. This top-tier performance highlights the test’s user-friendly design, allowing consumers to complete testing with fewer steps and clearer visuals compared to other products on the market. Many users report that the CareSuperb format feels more straightforward, reducing handling errors and providing reliable results in just 10 to 15 minutes.
“At a time when convenience and confidence matter most, the CareSuperb test delivers both,” said an Access Bio spokesperson. “Its ease of use helps ensure accurate testing in any household environment.”

Innovation Behind the Simplicity
Protected by U.S. patents, CareSuperb technology utilizes a proprietary adapter that prolongs the antigen-antibody reaction time, enhancing test sensitivity without complicating the process. This innovation allows consumers and healthcare providers to rely on laboratory-level accuracy from a home testing platform.
As a U.S.-based manufacturer, Access Bio is working to stabilize supply and prices amid increasing tariff pressures on imported medical products and pharmaceuticals. The company’s domestic production capabilities enable it to maintain competitive prices while ensuring reliable product availability throughout the winter months.
Strategic Retail Rollout
To support its retail launch, Access Bio has partnered with Market Performance Group (MPG)—a leader in omnichannel commerce and integrated brand growth. MPG’s team, composed of former executives from top retailers, CPGs, and digital platforms, is developing go-to-market strategies that connect Access Bio’s diagnostic innovations with consumers through mass, grocery, drug, club, specialty retail, and eCommerce channels.
“MPG’s expertise in retail activation and analytics will help us bring the CareSuperb test to households nationwide,” the company stated. “Together, we’re ensuring that consumers have access to high-quality diagnostic tools wherever and whenever they need them.”
Raising the Bar for Home Diagnostics
By combining proven accuracy with ease of use, Access Bio is setting a new standard for at-home testing. The CareSuperb Combo Kit enables individuals to take control of their health with a tool that’s both scientifically advanced and easy to use.
The company is confident that once consumers and healthcare professionals compare the CareSuperb Combo Kit to other at-home tests, the difference in ease of use and user experience will become immediately clear. Access Bio welcomes opportunities for direct comparative testing to highlight these advantages.
About Access Bio, Inc.
Founded in 2002 and headquartered in Monroe Township, New Jersey, Access Bio is a U.S.-based diagnostics manufacturer specializing in the development and mass production of testing solutions for infectious diseases, including malaria, dengue, influenza, COVID-19, and others. During the COVID-19 pandemic, Access Bio supplied over 300 million tests across the United States, playing a key role in the national public health response. Today, the company continues to innovate with accurate, affordable, and accessible diagnostic technologies that improve lives worldwide.