
CMS awards $50 billion to transform rural health nationwide
CMS made funding awards to all 50 states.

CMS made funding awards to all 50 states.
Activity is ramping up across most U.S. regions, with spikes in the Northeast and the Mountain West, as the new subclade K variant emerges and may affect population immunity.

The company has committed more than $70 billion in U.S. investments to boost domestic production and innovation.

Google Trends analysis revealed that new types of health-related searches emerged in the second half of 2025 and will continue into next year.

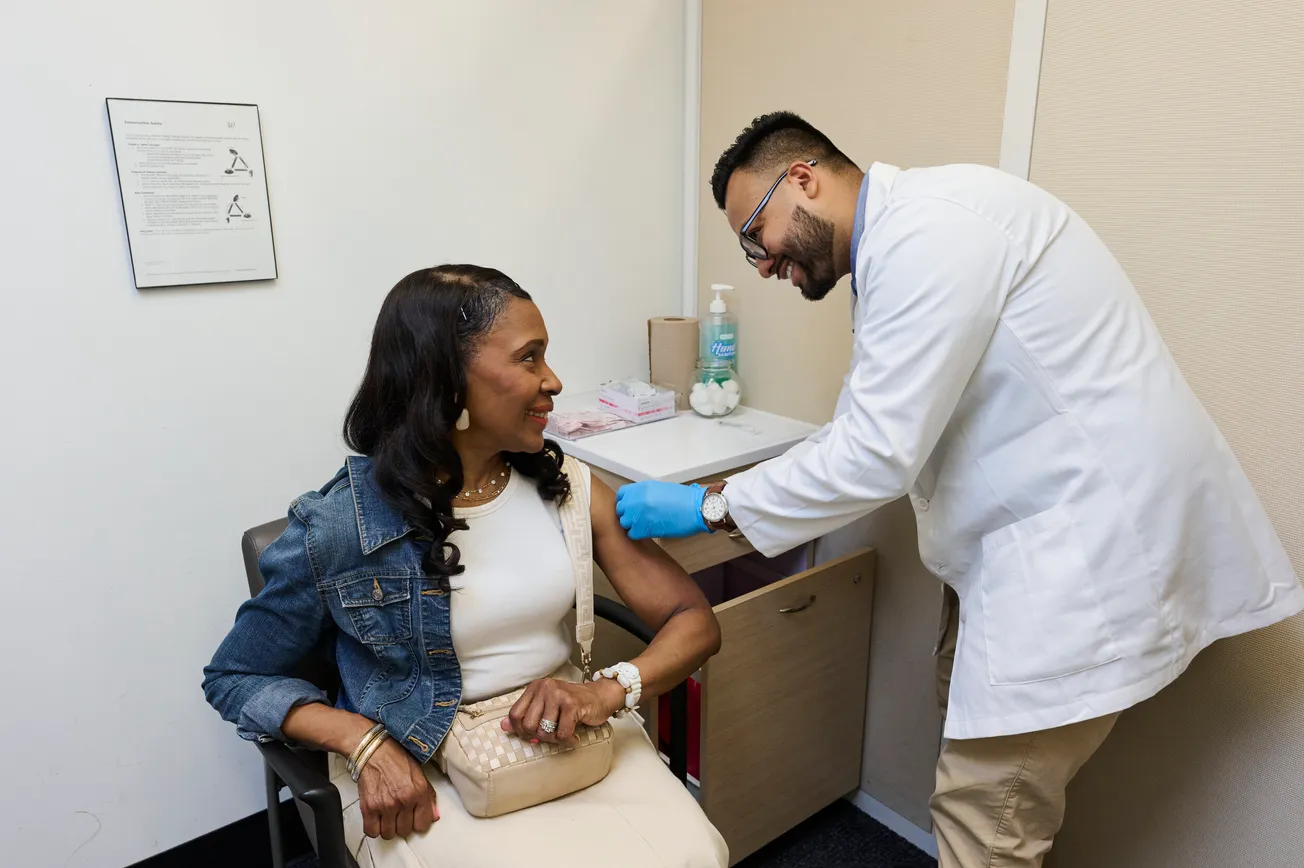
It marks the first time American clinicians will be able to prescribe a novel non-drug therapy called transcranial direct current stimulation (tDCS) for at-home use.

New additions continue to raise the bar for high-performance daily SPF.

The bills offer some of the most significant federal reforms to date aimed at lowering prescription drug costs, ending exploitative PBM practices, and protecting patient access to care.

Following an initial launch earlier this year, the program is now available nationwide to meet the growing interest from team members seeking to pursue PharmD degrees.

Target SoHo delivers a dynamic, continuously evolving assortment designed to keep pace with what's next.

At the end of the semester, the students presented their campaign concepts to the ear care product company's CEO, Elyse Stoltz Dickerson, and the eosera marketing team.

The micro-fulfillment center (MFC) will support nearly 96 stores across the region.

The suit, filed in California Superior Court, names Kraft Heinz, Mondelez, Post, Coca-Cola, PepsiCo, General Mills, Nestlé, Kellanova, Kellogg, Mars, and ConAgra.

Settlement resolves decade-long False Claims Act violations tied to premature refills and inaccurate reporting to federal health care programs.

Bundling medical procedures and pharmaceutical medications into one prior authorization.

Ultra-fast Amazon Now deliveries for household essentials and fresh groceries available to customers in some areas of Seattle and Philadelphia.

The company is seeking a full refund if the Supreme Court strikes down the bulk of President Trump's tariffs.