OXFORD – Owen Mumford, a global leader in medical devices, is pleased to announce that its exclusive agreement with Osaka-based Nipro Corp. to distribute drug delivery device UniSafe has achieved rapid early growth in combination product sales in Japan.
The agreement has been in place since 2019 and has scored a major new product launch success in delivering a biosimilar product into the Japanese market. This biosimilar is used with cancer medicines.
Since the launch of the product in late 2023, it has already acquired considerable share of its market in Japan, including the original biologic combination product. This success within just the first three months of commercial availability has exceeded expectations at NIPRO and Owen Mumford. Anecdotal feedback indicates this acceptance rate has been accelerated by the existing good reputation of UniSafe on the Japanese market, based on its ease of use and concealed needle.

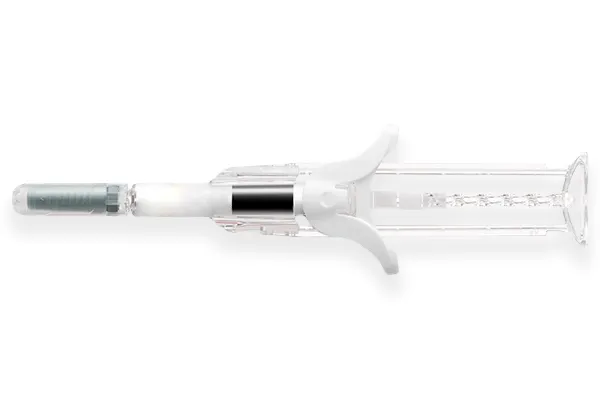
The biosimilar product is delivered through Owen Mumford’s UniSafe safety syringe. UniSafe is a unique springless, passive safety device for 1mL pre-filled syringes which has been designed to overcome some of the challenges of traditional spring-based safety systems. The absence of a spring means UniSafe is reliable, intuitive and easy to use. The design of the secure plunger helps to prevent re-use and accidental removal. UniSafe is also designed to prevent needlestick injury.
Masanobu Iwasa, Director PharmaPackaging Division at NIPRO, comments, “Japan has a particularly stringent regulatory environment, and so that makes our rapid and early success with the product all the more gratifying. Our careful development process, in tandem with our Owen Mumford partnership, has clearly appealed to clinicians and their patients alike. The product’s rapid take-up shows that we are meeting a true market demand.”
Tim Holden, Commercial Head, Pharmaceutical Services at Owen Mumford adds: “We are very committed to the Japanese market, with an established presence that has recently been greatly enhanced through our partnership with NIPRO over UniSafe. Healthcare in Japan is growing strongly, with healthcare spending rising by over a fifth between 2018 and 2025. In partnership with NIPRO, we intend to continue bringing innovative therapies to Japanese patients.”