
Marisa Tomei’s wellness brand Terra Mare gets gold at 2025 Nourish Awards
The actress and activist said the award reflects the brand’s mission.

The actress and activist said the award reflects the brand’s mission.

“Bold differentiation and cultural resonance will be table stakes for growth.”

“People will die,” said Senator Jon Ossoff, Democrat of Georgia.

“We are restoring SNAP to its true purpose – nutrition,” Agriculture Secretary Brooke Rollins said as she announced the waivers.
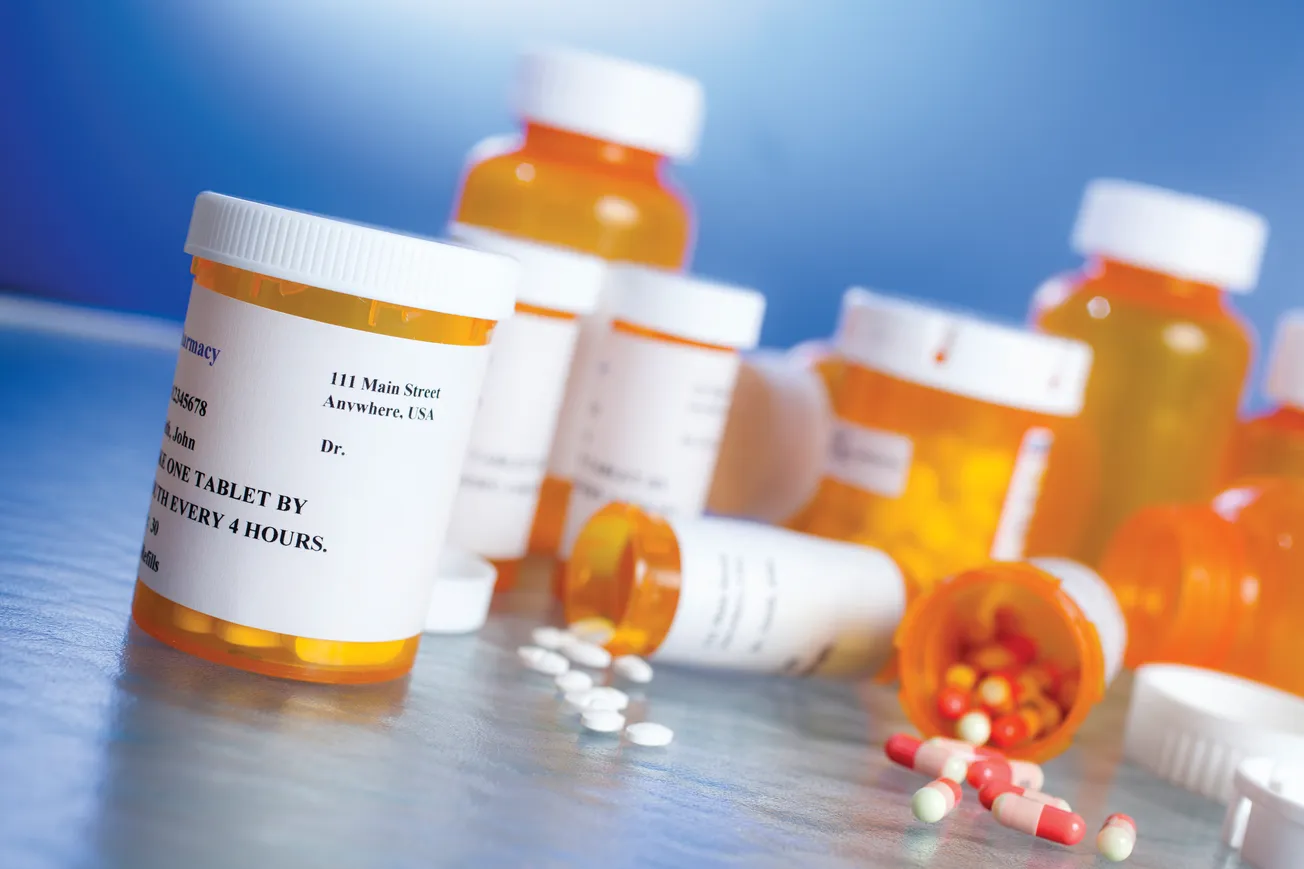
Too often we overlook the simplest, most dangerous fact: leftover prescriptions sitting in households are the fuel that sustains misuse, diversion, and tragedy.

Unvaccinated residents can receive a measles vaccine at select CVS Pharmacy and MinuteClinic locations across the state.

Over the last year, the top nine Canadian grocers grew two times more than grocers in the bottom ranking tercile.

SenderraRx's management team will remain significant investors and continue to lead the company.

Cardinal Health’s Global Medical Products and Distribution (GMPD) team provides essential solutions, including clinical diagnostic testing support, critical personal protective equipment (PPE) and safety-engineered solutions for vaccine administration.

The day reinforced Founders’ Innovation Day's mission: delivering content, building community, and creating connections that foster long-term success.

Williams brings over twenty years of experience in government affairs and public policy at both the state and national levels.

Success in today’s retail environment depends on clarity, customer focus, and a willingness to leverage data at scale.

Recent surveys show that one in three Americans now uses AI for some aspects of personal health management.

The event is expected to attract hundreds of pharmacy professionals, student pharmacists, and other allied advocates.

“He has made a significant impact on many aspects of our profession and is well-deserving of this recognition.”

Although social commerce still represents a small percentage of the retail ecommerce pie (6.9%), its share is steadily increasing.