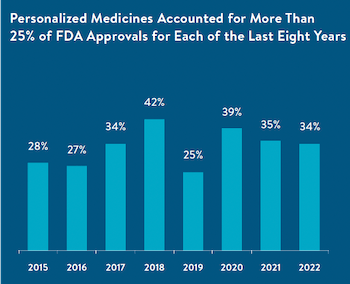
WASHINGTON — The Personalized Medicine Coalition (PMC) today released a report showing that personalized medicines designed for subpopulations of patients with certain biological characteristics accounted for 34% of the U.S. Food and Drug Administration (FDA)’s new drug approvals in 2022 and have accounted for at least 25% of approvals for each of the last eight years. Personalized medicines now account for more than a quarter of the new therapeutics approved since 2015. They have comprised more than a third of new drug approvals for five of the last six years.
Documenting the pharmaceutical industry’s shift away from one-size-fits-all drugs, the report, titled Personalized Medicine at FDA: The Scope & Significance of Progress in 2022, shows how the biopharmaceutical industry and FDA have pivoted toward personalized medicine in response to the emergence of new technologies and scientific discoveries related to biological markers that may influence the safety and efficacy of certain treatments. Despite high development costs and challenging reimbursement headwinds, the findings suggest that diagnostics and biopharmaceutical industry executives remain committed to personalized medicine based on its potential to improve patient care and make health systems more efficient by targeting treatments to only those who will benefit.
‘A Tremendously Significant Year’
The report classifies 12 of the 35 drugs approved by FDA’s Center for Drug Evaluation and Research as well as five gene and cell-based therapies approved by its Center for Biologics Evaluation and Research in 2022 as personalized medicines. It also explores the implications of new and expanded indications for 12 diagnostic testing platforms and examines how new diagnostic and therapeutic products may impact the care of certain patients with rare genetic diseases, cancers, and some common and infectious diseases.
“With milestones including the approval or clearance of new or expanded indications for three blood-based biomarker testing solutions that provide more testing options for cancer patients, five gene and cell-based therapies designed to address root genetic causes of some rare diseases and cancers, and a KRAS-targeted cancer treatment that shows how personalized medicine continues to redraw scientific boundaries, 2022 was a tremendously significant year for personalized medicine at FDA,” study author and PMC Senior Vice President for Science Policy Daryl Pritchard, Ph.D., said of this year’s analysis.
PMC Board Chairman and Culmination Bio CEO Lincoln Nadauld, M.D., Ph.D., who is also a practicing oncologist at Intermountain Health, added that “targeted therapies, cell-based immunotherapies, and liquid biopsy tests, as they are integrated into the standard of care for cancer patients, are becoming the hallmarks of a new era of personalized medicine in oncology.”
Continued Progress Cannot be Taken for Granted
Despite the positive signals, the report concludes that continued progress in personalized medicine cannot be taken for granted. To ensure that scientists and innovators continue to develop groundbreaking personalized medicine tests and treatments for the benefit of patients and health systems, the report contends that policymakers, as they have in the past, must support policies that encourage the advancement of the field.
“Personalized Medicine at FDA: The Scope & Significance of Progress in 2022 underlines the pharmaceutical industry and FDA’s unsung commitment to personalized medicine, which has important implications for the future of patient care,” said PMC President Edward Abrahams.









