
Cain’s Drug Store named IPC 2023 Most Valuable Pharmacy
Independent Pharmacy Cooperative (IPC) announced that Cain’s Drug Store of Vandalia, Ill., as the recipient of the Dan Moudry Most Valuable Pharmacy (MVP) for 2023.

Independent Pharmacy Cooperative (IPC) announced that Cain’s Drug Store of Vandalia, Ill., as the recipient of the Dan Moudry Most Valuable Pharmacy (MVP) for 2023.
EAST WINDSOR, N.J. – Aurobindo Pharma Limited has received final approval from U.S. Food and Drug Administration for its Abbreviated New Drug Application for Aurobindo Receives FDA Approval for rufinamide tablets USP, 200mg and 400mg.

Pharmsource has joined forces with LSPedia, a leading provider of cloud-based supply chain solutions, in a groundbreaking partnership that advances compliance for pharmacies by expanding their access to vital compliance solutions.

SPARTANBURG, S.C. –RedSail Technologies, developer of Axys, announced its integration with Eldermark, a leading provider of senior living software. This collaboration delivers enhanced care, operational efficiency, and improved financial outcomes for senior living communities.

McKesson announces the launch of Foster & Thrive, a curated private brand of over-the-counter (O-T-C) health and wellness products.

ARLINGTON, Va. – The National Association of Chain Drug Stores (NACDS) today praised the bipartisan introduction of the Protect Patient Access to Pharmacies Act (S.

ARLINGTON, Va. – Organizations representing pharmacists and pharmacies are applauding the Protect Patient Access to Pharmacies Act (S. 2052), introducedtoday in the U.S. Senate.
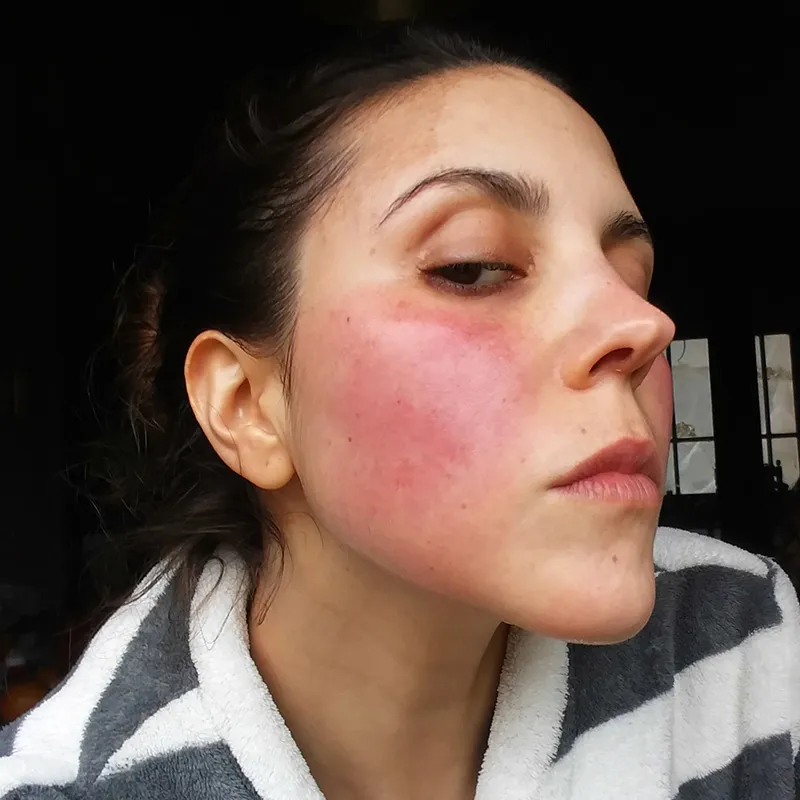
The National Rosacea Society (NRS) today announced that it is launching a Seal of Acceptance program to identify skin care and cosmetic products that may be suitable for people who suffer from rosacea.

The Pharmaceutical Care Management Association (PCMA) released the following statement today ahead of JC Scott, president and CEO of PCMA, testifying before the U.S.

The National Association of Specialty Pharmacy (NASP) announced the full agenda for the NASP 2023 Annual Meeting & Expo. The conference will take place September 18 – 21, 2023 at the Gaylord Texan Resort & Convention Center in Grapevine, Texas.

DocStation, a leading provider of innovative pharmacy software solutions, announces the launch of its groundbreaking Auto-Billing functionality, a revolutionary feature that transforms revenue streams for pharmacies.

The White House announced that President Biden will name Dr. Mandy Cohen, a former North Carolina official, to be the new director of the Centers for Disease Control and Prevention.

Aetna Better Health of Georgia, a CVS Health, announced that Aetna provided a community investment of $140,000 to the Morehouse School of Medicine. The investment will support Morehouse School of Medicine’s Health Equity for All Lives (H.E.A.L.

ALEXANDRIA, Va. – Registration is now open for the National Community Pharmacists Association’s 2023 Annual Convention, set for Oct. 14–17, in Orlando. NCPA president Hugh Chancy says it’s the premier event for community pharmacy and a can’t-miss meeting.

Cardinal Health announced Thursday its plans to build a new distribution center in the Greenville, S.C., area, to support its at-Home Solutions business, a market-leading home healthcare medical supplies provider serving people with chronic and serious health conditions in the United States.

U.S. Rep. Max Miller (R., Ohio) visited Discount Drug Mart’s corporate headquarters and distribution center, and met with Don Boodjeh, the retailer’s CEO, and other company executives.