
CMS launches landmark $50 billion Rural Health Transformation Program
New federal program aims to transform rural health care nationwide.

New federal program aims to transform rural health care nationwide.

With more than 2,100 attendees gathered in Denver, the NASP 2025 Annual Meeting & Expo provided the perfect stage to spotlight and celebrate this year’s distinguished Industry Award winners.

Two Republican congressmen are looking at CVS over concerns that the company used confidential patient information to lobby against a Louisiana bill that would have forced it to break up its pharmacy business.
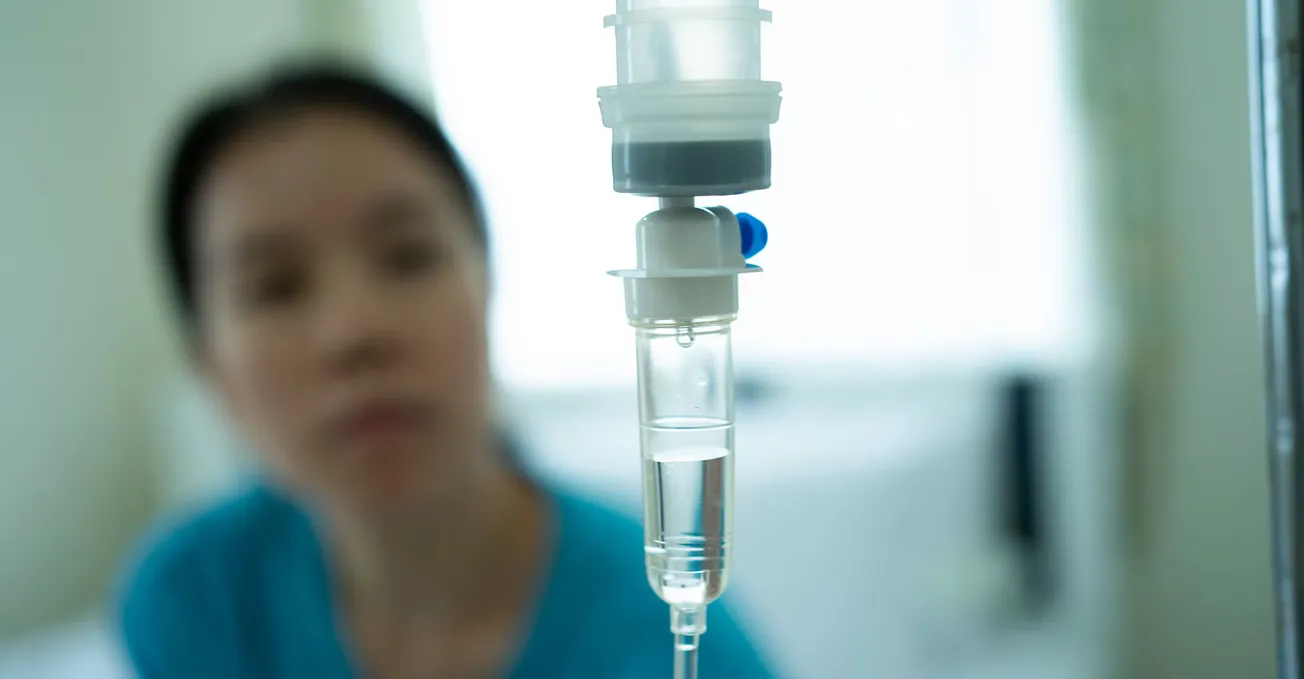
CVS Infusion Care locations are said to provide a safe, cost-effective and convenient setting for eligible patients.

As the agency opens its books and debate intensifies, the future of U.S. vaccine policy and public confidence may depend on how the next week unfolds.

Marcus Lanznar has been named president of Signify Health, and Jon Thiboutot has been named president of retail health for CVS Health.

Paytient members can now see transparent, low-cost cash prices for medications in their app, making it easier to save money and time.

Sodium oxybate oral solution is a central nervous system depressant indicated for the treatment of cataplexy or excessive daytime sleepiness in patients 7 years of age and older with narcolepsy.

“Dr. Collier’s decades of dedication, vision, and leadership embody the spirit of this award,” said CHPA President and CEO Scott Melville.

Emma Mittelstaedt Burnham is an experienced antitrust enforcer, exactly what patients and providers need to go up against PBMs and insurers.

CatalinaRx combines deterministic purchase data and predictive audience modeling with the nationwide addressable retail print media network.

The report aims to improve children’s health, but critics say it relies on flawed science and weak regulation.

The patented solution secures prescriptions from pharmacy to medicine cabinet.

“Current research has not established a causal link between acetaminophen use and adverse neurodevelopmental outcomes.”

Time to pass bipartisan measures, say 134 groups.

Project affirms commitment to supporting the domestic pharmaceutical industry and strengthens pharmacy supply chain.