Pharmaceutical traceability is here.

Lari Harding
Are you ready? As an industry we have been working for 10 years to improve the quality and safety of the drug supply chain with the promise that serialization will prevent illegitimate, counterfeit, substandard and unapproved drugs from reaching patients. However, industry reports show that readiness is a mixed bag across stakeholders.
In 2013 Congress signed into law the Drug Supply Chain Security Act (DSCSA), replacing a state-level patchwork of pedigree requirements with one federal solution. By 2015 we began transacting only with licensed and registered trading partners. In 2017 manufacturers introduced serialized product identifiers, followed by repackagers in 2018.
In 2019 distributors were to begin transacting only serialized products and performing verification of saleable returns and, in 2020, dispensers were to begin transacting only serialized products. But COVID-19 brought exemptions, and the FDA postponed enforcement until 2023.
So, now, here we are. By November 27, 2023, it is expected that the entire pharmaceutical supply chain will track unit-level traceability, an interoperable electronic system will be in place throughout the entire supply chain, and saleable returns will need to be verified. Within this system, pharmacies are expected to scan and validate every product that comes into their pharmacy.
Who knew, 10 years ago, that we would be facing the kind of labor shortages we have today? According to the U.S. Chamber of Commerce, there are more than 10 million job openings in the U.S., but only 5.7 million unemployed workers. We have nearly 3 million fewer Americans participating in the labor force today compared to February 2020. In fact, labor force participation rates are the same as they were 45 years ago.
Faced with the same staffing challenges plaguing the rest of health care, some retail pharmacies, including large chains, have been forced to reduce their operating hours. The unit-level verification requirements are adding to those challenges. However, implementation of DSCSA guidelines with aggregation and inference using barcodes will help mitigate some staffing issues and improve efficiency in the data collection process.
The most important benefit from DSCSA is patient safety, which will be delivered by enabling prompt response to suspect and illegitimate products, improving the efficiency of recalls and creating transparency and accountability in the drug supply chain. It is estimated that approximately 2.5% of drugs in the supply chain are counterfeit, which is down from 3%.
In preparing for this year’s NACDS Annual Meeting, I thought a lot about the patient safety mission. Even more timely was a book I received over the holidays, Demon Copperhead by Barbara Kingsolver. A modern day retelling of the Charles Dickens classic, David Copperfield, it is a must-read for everyone in the pharmaceutical industry.
As does the original, this novel candidly presents the damaging effects of institutional poverty on children. While the main character, Demon, doesn’t have enough to eat, or clothes to wear, pharmaceutical drugs are easily accessible. At one point in the story, Demon says, “I don’t know a single person my age that’s not taking pills.” And he tells of “pharm parties” where he was first introduced to pills. It is a powerful message for effective drug stewardship that we must all hear.
Drug stewardship is escalating
Ensuring the proper disposal of unneeded medications is a patient safety issue that we must continue to address. According to a recent Inmar survey of 1,000 consumers, 75% of consumers have expired drugs in their homes. Another 56% admitted to using the sink or toilet to dispose of medications. When asked about the use of consumer drug take-back receptacles, 71% of people who reported using one said they did so because it was “convenient.” Only 17% said because it was “the right thing to do.”
There are similarities between DSCSA and drug stewardship. There is currently a patchwork of states, counties and municipalities with drug stewardship regulations that both manufacturers and pharmacies must comply with to remove unused medicines from consumers’ homes. Sounds similar to the state-level patchwork of pedigree laws that preceded federal regulation on pharmaceutical traceability.
When an industry voluntarily manages these challenges, the need for regulations diminishes. We have a number of voluntary heroes in our industry, including Meijer. In 2019 they put take-back receptacles in all of their stores, not because they were operating in regulated states, but because it was the right thing to do. It’s a move that’s not only good for the community but one that’s also good for business, as our research finds that 55% of pharmacy patrons would consider changing pharmacies if their pharmacies offered drug take-back.
At the same time, voluntary stewardship programs share the cost across industry participants. In regulated states the number of receptacles is significantly higher than in nonregulated states. We can do better to address this need in all states. This is but one of the many issues our industry is facing. Each issue has its own struggles, but the overarching goal is to achieve fair and adequate reimbursement so that pharmacies remain viable and deliver high-quality, accessible health care.
PBM reform is critical to the survival of retail pharmacy
There are similarities between DSCSA, drug stewardship and pharmacy benefit manager reform. The Supreme Court ruling in Rutledge v. Pharmaceutical Care Management Association paved the way for states to legislate on the issue of PBM reform. That’s critically important, as retail pharmacies continue to be challenged financially by growing fees charged by PBMs and declining reimbursement rates.
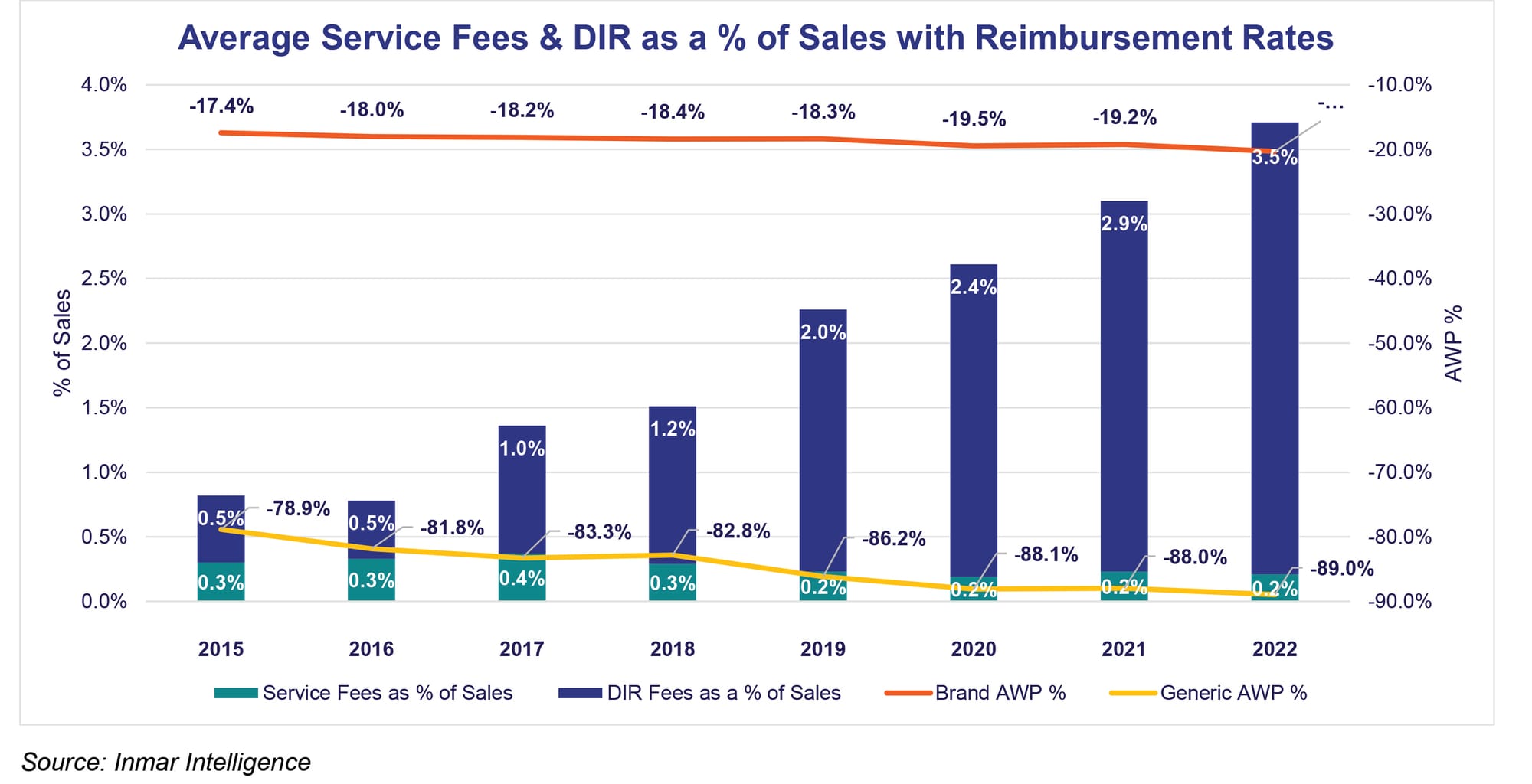
According to Inmar data, direct and indirect remuneration (DIR) fees averaged 3.5% of total sales in 2022. In 2015 and 2016 they were 0.5% of total sales. The intention was for DIR to be about quality and value-based patient care, but the result has been transferring pharmacy profits to PBMs. At the same time average reimbursement rates have declined.
PBMs have exacerbated this issue by taking a controlling stake in specialty pharmacy and by instituting drug pricing practices that have consumers paying 100% of the cost of prescriptions for 40% of the fills. This is driving the growth of cash-only and online pharmacy disruptors that have doubled their market share in the last year while the percentage of consumers that also use traditional pharmacies is dropping.
COVID-19 vaccinations helped with profitability over the last couple of years, but that is diminishing as the PBMs have started managing COVID vaccinations post-pandemic. Since 2020, there have been more than 100 new PBM reform laws enacted at the state level. Sounds similar to the state-level patchwork of pedigree laws that preceded federal regulation on pharmaceutical traceability.
Is the march toward quality and safety an opportunity?
What is your quality and safety story, and how are you telling it? Do you measure your quality and safety? Do you benchmark yourself against others? Do you advertise your quality and safety so that patients are aware? Do your pharmacists practice it in their interactions with patients? Do you have compelling data to share with legislators?
The retail health care market is expected to double in 2023, and many people believe that the future of primary care will be in retail — and in pharmacies. According to a recent survey sponsored by Wolters Kluwer, 61% of Americans think that pharmacies, retail clinics or pharmacy clinics will provide most primary care in the next five years. At the same time, a Bain & Co. study shows that new models of primary care will capture 30% of the U.S. market by 2030 as retailers, payer-owned providers and advanced primary care disruptors gain traction.
Connecting all of these industry dynamics — illegitimate products, labor shortages, preventable errors, the opioid crisis, rising health care costs, inefficiencies, disruptive business models — it is evident that we are in the midst of a health care crisis. As a nation we spend more on health care than any other country, but we do not have better outcomes. Barbara Kingsolver’s story demonstrates this as especially challenging for those attempting to escape institutional poverty and drug addiction in the richest country on Earth.
As we gather at NACDS Annual, let’s talk about how we can move from quality and patient safety as “compliance” to improving quality and safety as being a driver of excellence and business success. Let’s talk about traceability, drug stewardship and PBM reform as it relates to your strategies for achieving excellence in health care. Make your story about quality and safety. It’s a winning story.
Lari Harding is senior vice president of sales and marketing enablement at Inmar Intelligence.









