
Sai Life Sciences set to double process R&D capacity with New Hyderabad facility
The new facility will double the company’s Process R&D capacity, while adding new capabilities.

The new facility will double the company’s Process R&D capacity, while adding new capabilities.

Brand uncovers that 80% of consumers would love more IRL group chat meetups.

The retailer is stepping up as the go-to holiday partner, bringing style, joy and unbeatable value to guests and communities this season, both in store and online.

CVS Pharmacy continues to partner with Food Allergy Research & Education (FARE) as the exclusive pharmacy retail sponsor of the Teal Pumpkin Project.

The integrated campaign celebrates the spirit that defines both Coca‑Cola and the Olympic and Paralympic Games – uniting fans across the country in shared moments of pride, connection, and refreshment.
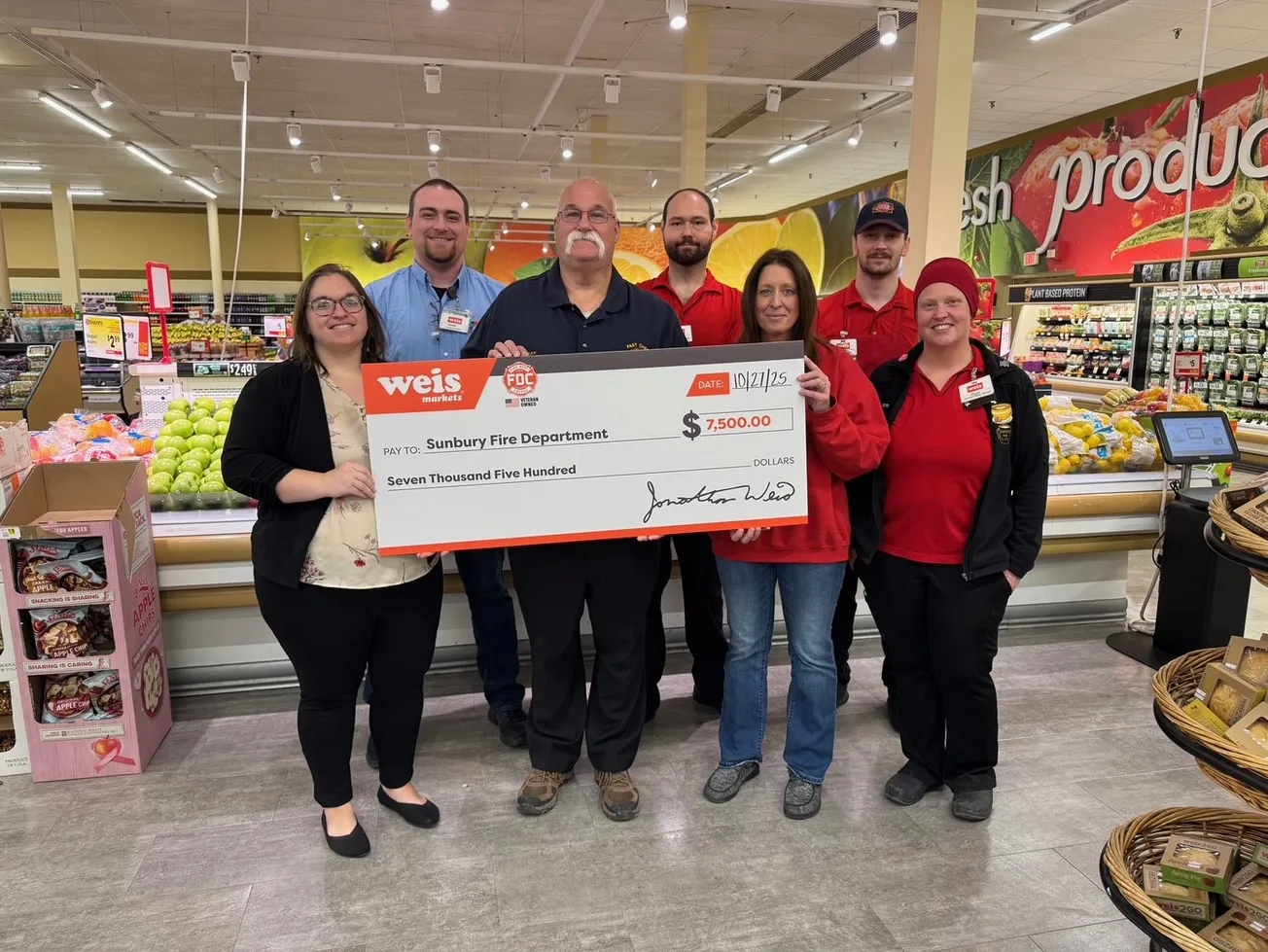
Weis Markets, in partnership with Fire Department Coffee, presented a $7,500 donation to the Sunbury Fire Department.

"We once again urge Congress to act immediately to restore government funding, provide clarity and food for families in need, and ensure that these vital programs remain dependable for those who have to rely on them to get them through a difficult time."

FDA Commissioner Makary joined AAM’s GRx+Biosims 2025 conference today.

In a new draft guidance, the FDA proposes major updates to simplify biosimilarity studies and reduce unnecessary clinical testing.

Morning After Pill is now the first and only EC widely available in brick-and-mortar retailers for under $30.

SHARx operates on a transparent pricing model—charging a simple subscription fee.

Adjusted earnings per share of $1.60 topped Wall Street's forecast of $1.37, while revenue of $102.87 billion exceeded the predicted $98.85 billion.

Walmart will serve as the first in-store pickup pharmacy for LillyDirect’s self-pay single-dose vials.

Sri recaps his visit to Amazon Accelerate.

Eli Lilly announced its Team USA lineup for the 2026 Winter Games. The company will also expand its Milestones into Meaning program.

The expansion advances Padagis’ multi-year plan to onshore vital medication production.