NEW YORK — Pricing pressures have become the new normal for the pharmaceutical industry. Three main factors are forcing down drug prices: government pressure, patent losses and the rise of generics.

Giedre Liorancaite
In developed markets, governments account for nearly 76% of spending on hospitals and other medical services. As a result, rising government measures to curb health care costs will continue to be manifest in terms of regulatory pricing pressures for the pharmaceutical industry.
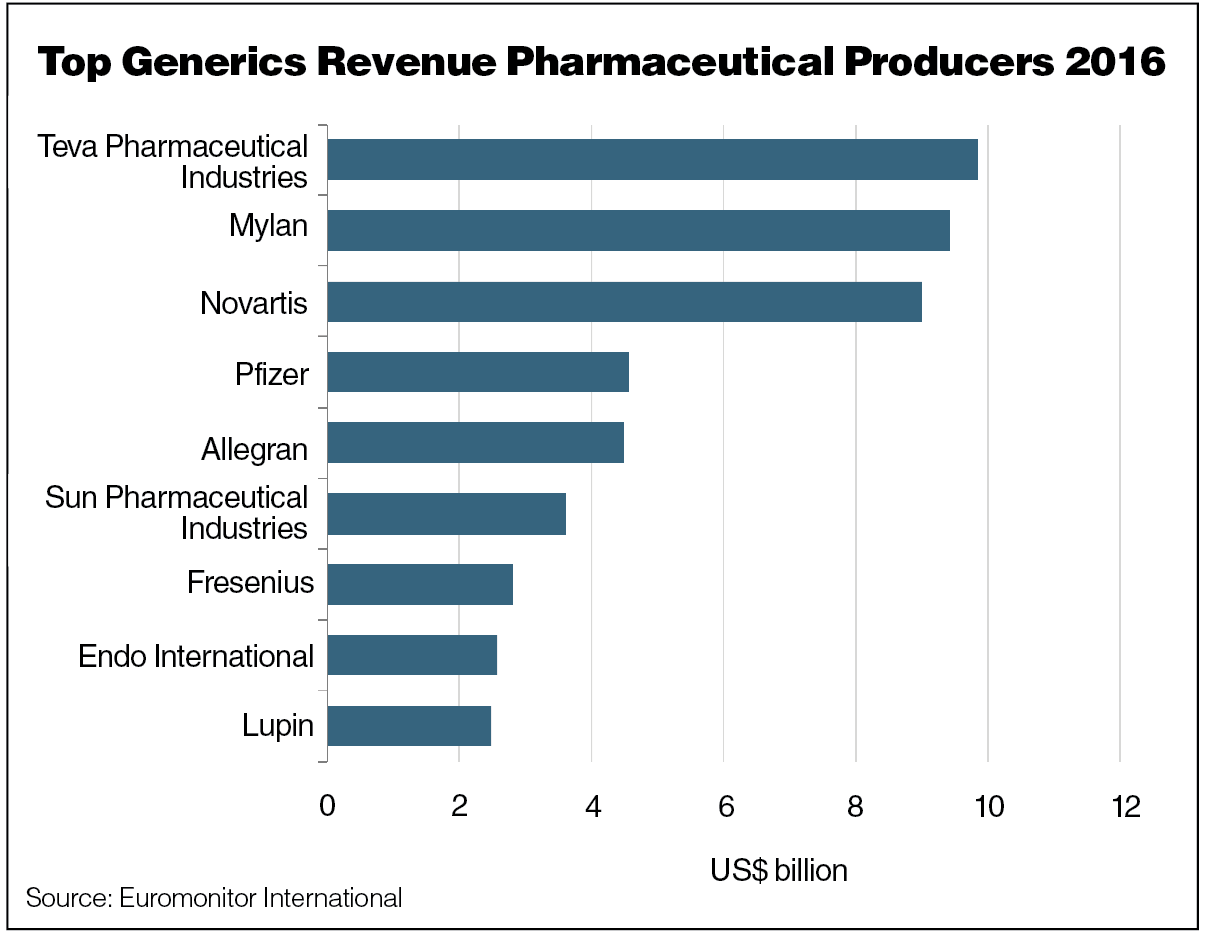
Second, pending upcoming patent expirations, drug manufacturers will be unable to hold the initially high prices for their branded products. In 2016 alone, key pharmaceutical companies collectively lost around $10 billion in revenue thanks to loss of exclusivity. Finally, in countries like Italy, the generics market already makes up 80% of volume sales, and the consumption of generics and biosimilars is anticipated to continue to increase in the near future.
Governments to launch new pricing policies
Health care service providers are by far the largest B2B buyers of pharmaceuticals, accounting for 32% of total market sales. While government spending on medicines is slowing, the number of people in need of treatment is rising, especially among the elderly. Increasing numbers of patients in hospitals, coupled with rising public debt, are expected to underpin sustained government measures to curb health care expenditure. One such way is to put further regulatory pressure on pharmaceutical prices, as publicly owned hospitals are the key buyers of drugs.
Developed countries, in particular, are creating new policies on the prices of both generic and name brand medicine.
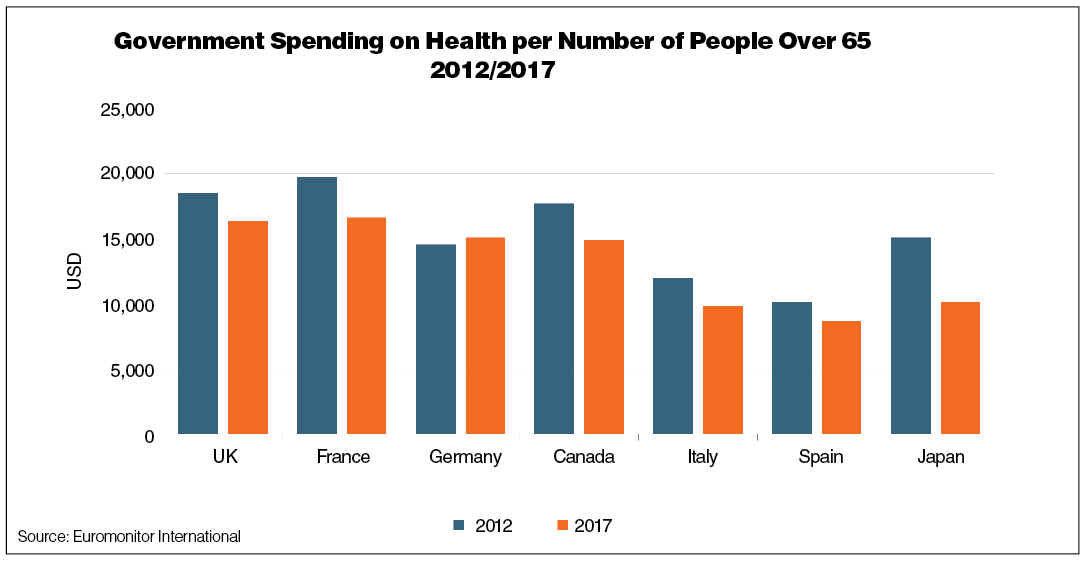
• United Kingdom: The U.K. introduced cost caps for pharmaceuticals that are reimbursed by the National Health Service. The government also created the Health Service Medical Supplies Act, which grants public authorities more power to regulate medicine prices.
• France: The government’s efforts to regulate prices intensified in 2016: The prices received by French medicine producers fell by 4% during the year. The government signed an agreement with the Federation of Medicines Enterprises to regulate the price of medicines from 2017 to 2019.
• Germany: In 2017, the government approved the German Drug Law (AM-VSG), aiming to ensure Social Health Insurance financial stability and extend the price moratorium for all patent-free drugs until 2022.
• Italy: The budget ceiling for outpatient pharmaceutical expenses was set at 11.35% of the National Health Fund from 2013 to 2016 and is planned to be further tightened to 7.96% in 2017.
• Japan: Toward the end of 2016, the government launched a reimbursement pricing system reform, which introduces annual drug repricing; the new policy is set to most severely affect the pricing of generics.
Company revenues negatively affected by patent losses
Many key drug companies engaged in production of branded pharmaceuticals have recently reported revenue losses, due to patent expirations of key blockbuster drugs and, as a consequence, declining drug prices and market sales. Around two dozen patents expired in 2016, including such pharmaceuticals as AstraZeneca PLC’s cholesterol drug Crestor (representing $3.4 billion worth of revenue), Daiichi Sankyo’s blood pressure drug Benicar ($2.6 billion) and Merck & Co.’s cholesterol drug Zetia ($2.6 billion). The trend was set to continue in 2017, as 18 drugs faced patent expirations that year.
Increased promotion of generics and biosimilars
The high price of biologics and novel treatments is expected to lead governments to promote the use of generics and biosimilars, putting further pressure on prices. The countries with mainly publicly financed health care systems, including as Canada, Germany, Denmark and the Netherlands, have introduced various incentive programs to facilitate the consumption of equivalent cheaper versions of branded drugs. Biosimilar market growth is expected to be further facilitated by the loss of patent protection for best-selling biologics, including Humira (rheumatoid arthritis), Remicade (autoimmune diseases) and Lantus (diabetes), opening the way for generics to enter the market and increase competition among producers of biologics.
Competition will increase with new Asian entrants
The competition in biologics is expected to further intensify as Asian companies like Celltrion, Samsung BioLogics and Dr. Reddy’s are aiming to aggressively enter the global market and challenge Big Pharma.
For example, Celltrion has spent 30% to 40% of revenue on R&D and plans to capture half of the global infliximab biosimilars market. With its aggressive market penetration tactics, the company has already captured significant share in key biologics markets in Europe. The company reported that its drug Remsima (autoimmune disease) was able to seize around 30% of the original drug sales in the year after it was launched. Meanwhile, the biologic Truxima (blood cancer) was able to secure over one-third of the original drug market in key markets, such as the U.K. and the Netherlands, in just three months after its official launch in April 2017.
Overall, drug prices in developed markets will remain under pressure, in line with government-mandated initiatives, expected patent losses and rising generics penetration. Key players are already losing profits and, as a result, are ramping up advertising budgets and externalizing more R&D activities. However, in the longer run, companies can focus on caring for older consumers and employing new technology, either to reduce their manufacturing costs or to improve their products.
Giedre Liorancaite is a senior industry analyst at Euromonitor International. She can be reached at Giedre.Liorancaite@Euromonitor.com









