
Dr. Reddy’s launches first-to-market OTC generic of extra-strength Pataday in U.S.
“The first-to-market launch underscores our capabilities in bringing store-brand equivalents of leading OTC brands to the U.S. market.”

“The first-to-market launch underscores our capabilities in bringing store-brand equivalents of leading OTC brands to the U.S. market.”

Manufactured in Texas at one of Extrovis’ facilities, Dr. Reddy’s Fluorouracil Cream, 0.5%, is indicated for the topical treatment of multiple actinic or solar keratoses of the face and anterior scalp.
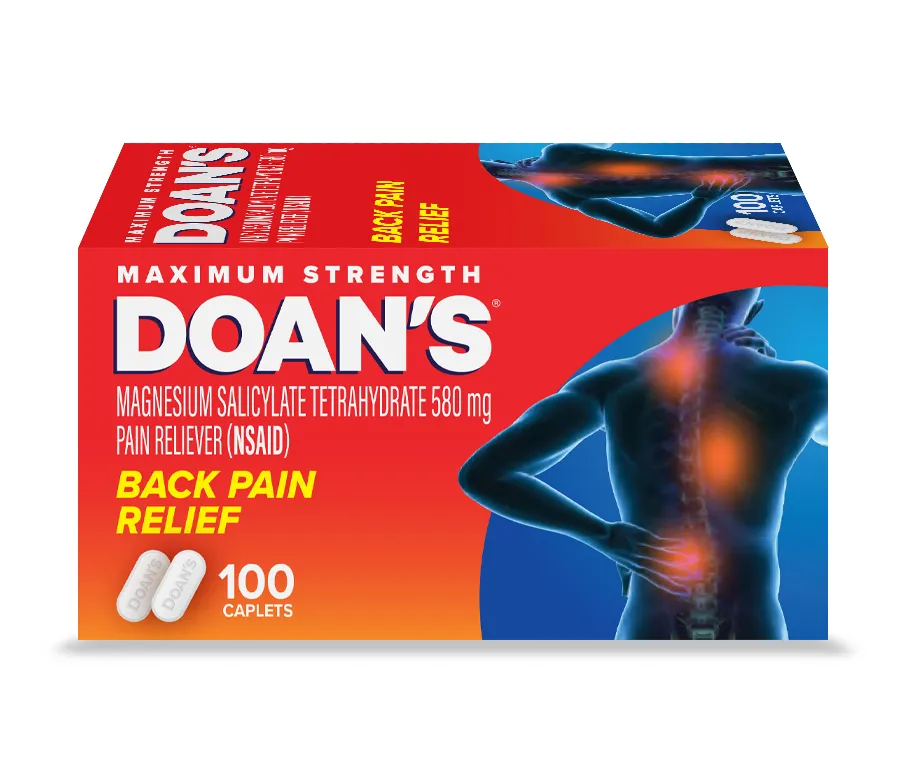
“We are pleased to launch the 100-count — we know back pain affects many adults of all ages,” said Kathryn Healy, head of marketing for Doan’s for manufacturer Dr. Reddy’s.

Girota’s role involves shaping the strategic road map and scaling the business with the aspiration to establish Dr. Reddy’s as a leading player in the consumer health segment

The collaboration combines Dr. Reddy’s and Alvotech’s proven capabilities in biosimilars, thus, speeding up the development process and extending the global reach for this biosimilar candidate.

This marks the third consecutive appearance of Dr. Reddy’s in the list.